Abstract
Introduction:
A number of cytogenetic abnormalities (CAs) are associated with poorer prognosis in MM, including del(17p), t(4;14), t(14;16), and amp1q21. There is a general consensus that treatment with proteasome inhibitors (PIs) benefits pts carrying these CAs (Sonneveld Blood 2016). This meta-analysis of four phase 3 studies assesses PFS benefit in pts receiving the oral PI ixa vs pbo regarding the specific adverse CAs.
Methods:
Pts in TOURMALINE-MM1 (N=722; relapsed/refractory MM; Moreau N Engl J Med 2016) and MM2 (N=705; newly diagnosed MM; Facon Blood 2021) received ixa plus lenalidomide-dexamethasone (Rd) vs pbo-Rd (1:1). Pts in TOURMALINE-MM3 (N=656; Dimopoulos Lancet 2019) and TOURMALINE-MM4 (N=706; Dimopoulos J Clin Oncol 2020) received ixa vs pbo (3:2) as maintenance following autologous stem cell transplant or as post-induction maintenance in transplant-ineligible pts, respectively. In TOURMALINE-MM1/MM2, CAs were centrally assessed on CD138 positive sorted cells from bone marrow samples collected at study entry using fluorescence in situ hybridization (FISH). Cutoff values for defining the presence of del(17p), t(4;14), and t(14;16) were 5%, 3%, and 3% positive cells, respectively, based on the false-positive rates (technical cutoffs) of the FISH probes used, and cutoff values of 3% (MM1) and 20% (MM2) were used for amp1q21. In TOURMALINE-MM3/MM4, cytogenetic assessment was performed locally using FISH or conventional karyotyping with locally defined thresholds for positivity.
Results:
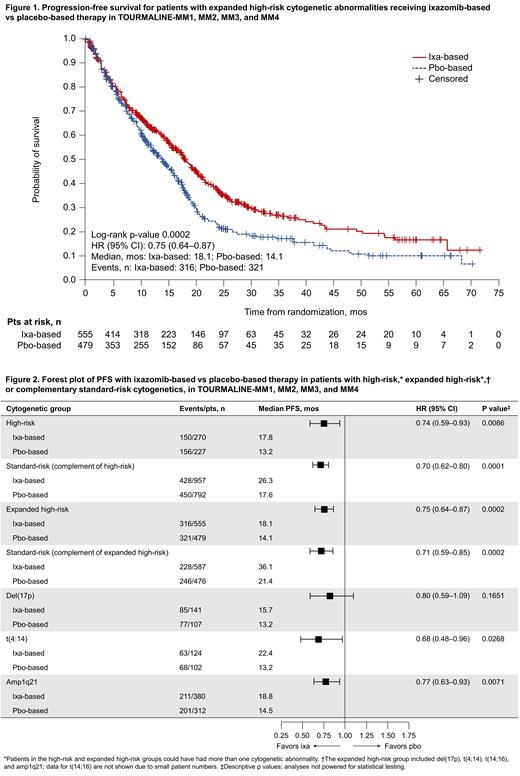
270/1227 (22%) vs 227/1019 (22%) evaluable pts receiving ixa-based vs pbo-based therapy had high-risk CAs [del(17p), t(4;14), t(14;16)]: 75 vs 62 in MM1, 60 vs 63 in MM2, 61 vs 54 in MM3, and 74 vs 48 in MM4. 957/1227 (78%) vs 792/1019 (78%) had complementary standard-risk CAs: 200 vs 216 in MM1, 231 vs 234 in MM2, 252 vs 152 in MM3, and 275 vs 190 in MM4. 555/1142 (49%) vs 479/955 (50%) evaluable pts receiving ixa-based vs pbo-based therapy had expanded high-risk CAs (high-risk CAs ± amp1q21): 155 vs 154 in MM1, 134 vs 146 in MM2, 116 vs 88 in MM3, and 150 vs 91 in MM4. 587/1142 (51%) vs 476/955 (50%) had complementary standard-risk CAs: 122 vs 126 in MM1, 164 vs 153 in MM2, 154 vs 89 in MM3, and 148 vs 108 in MM4.
After a median follow-up in the pooled analysis of 25.6 months (mos; 12.7, 54.6, 29.7, and 21.3 mos in MM1, MM2, MM3, and MM4, respectively), the hazard ratio (HR) for PFS with ixa-based vs pbo-based therapy in pts with high-risk CAs was 0.74 (95% confidence interval [CI] 0.59-0.93; median 17.8 vs 13.2 mos) and 0.70, (95% CI 0.62-0.80; median 26.3 vs 17.6 mos) in pts with standard-risk CAs. In the subgroup analyses of expanded high-risk CAs, the HR for PFS with ixa-based vs pbo-based therapy in pts in the expanded high-risk group was 0.75 (95% CI 0.64-0.87; median 18.1 vs 14.1 mos; Figure 1) and 0.71 (95% CI 0.59-0.85; median 36.1 vs 21.4 mos) in the complementary standard-risk group. Analyses of PFS according to the presence of individual CAs (Figure 2) indicated differing magnitudes of PFS benefit. Notably, in pts with t(4;14) (n=124 vs n=102), the HR for PFS with ixa-based vs pbo-based therapy was 0.68 (95% CI 0.48-0.96; median 22.4 vs 13.2 mos), while for pts with amp1q21 (n=380 vs n=312), the HR was 0.77 (95% CI 0.63-0.93; median 18.8 vs 14.5 mos) and for pts with del(17p) (n=141 vs n=107) the HR was 0.80 (95% CI 0.59-1.09; median 15.7 vs 13.2 mos).
Conclusions:
This pooled analysis demonstrated a PFS benefit with ixa-based therapy vs pbo-based therapy regardless of the presence of specific adverse CAs, with a similar magnitude of benefit in pts with (expanded) high-risk CAs and the respective complementary standard-risk groups. However, due to the differences in study eligibility criteria and pt populations, ixa combined with Rd or as single-agent maintenance therapy may not abrogate the negative impact of high-risk CAs. Analyses of PFS in subgroups with specific CAs indicated that the greatest magnitudes of benefit (lowest HRs) with ixa-based vs pbo-based therapy were in pts with t(4;14) (HR 0.68) and pts with amp1q21 (HR 0.77), suggesting that the improved outcome with ixa-based vs pbo-based therapy in the expanded high-risk subgroup was primarily driven by PFS differences in pts with these more common CAs. Further study is needed, and additional sensitivity analyses will be presented in subsequent publications.
Chng: BMS/Celgene: Honoraria, Research Funding; Amgen: Honoraria; Takeda: Honoraria; Abbvie: Honoraria; Sanofi: Honoraria; Pfizer: Honoraria; Johnson and Johnson: Honoraria, Research Funding. Lonial: BMS/Celgene: Consultancy, Honoraria, Research Funding; AMGEN: Consultancy, Honoraria; GlaxoSmithKline: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria. Morgan: Takeda: Honoraria. Iida: Amgen: Research Funding; Daiichi Sankyo: Research Funding; Glaxo SmithKlein: Research Funding; Ono: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Chugai: Research Funding; Abbvie: Research Funding; Janssen: Honoraria, Research Funding; Bristol-Myers Squibb: Research Funding. Moreau: Sanofi: Honoraria; Celgene BMS: Honoraria; Abbvie: Honoraria; Amgen: Honoraria; Janssen: Honoraria; Oncopeptides: Honoraria. Kumar: Bluebird Bio: Consultancy; Carsgen: Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Antengene: Consultancy, Honoraria; Novartis: Research Funding; Oncopeptides: Consultancy; Tenebio: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche-Genentech: Consultancy, Research Funding; Beigene: Consultancy; Merck: Research Funding; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding. Twumasi-Ankrah: Takeda: Current Employment. Kumar: Takeda: Current Employment, Current holder of stock options in a privately-held company. Dash: Takeda: Current Employment, Current equity holder in publicly-traded company. Vorog: Takeda: Current Employment. Zhang: Takeda: Current Employment. Suryanarayan: Takeda: Current Employment. Labotka: Takeda: Current Employment. Dimopoulos: Janssen: Honoraria; Takeda: Honoraria; Beigene: Honoraria; BMS: Honoraria; Amgen: Honoraria.
Use of the oral proteasome inhibitor ixazomib for the initial treatment of multiple myeloma and as maintenance treatment following stem cell transplantation or induction therapy in newly diagnosed patients

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal